Pseudogene Function: More Evidence by John Woodmorappe
According to standard evolutionary thinking, pseudogenes are simply disabled copies of genes. Arguments for shared evolutionary ancestry have been advanced based on the similarities in perceived disablements found in orthologous pseudogenes (counterpart pseudogenes in other primates).1 However, a close examination shows that this presumed evidence is equivocal. Dissimilarities between the pseudogenes of presumably related organisms are at least as prominent as the similarities, and similarities in orthologous pseudogenes can arise independently of shared evolutionary ancestry.2
In addition, arguments for shared evolutionary ancestry assume that pseudogenes lack function, and so would not have been specially created with a series of shared similarities from organism to organism. This too is increasingly open to question. Pseudogenes of protein-coding genes are usually compared with their certainly-functional gene paralogs (gene copies within the same organism), and inferences are made about lack of function based on deviations in sequence that are perceived to prevent the eventual synthesis of a functional peptide. However, as elaborated elsewhere,3 the distinction between functional and nonfunctional gene copies is becoming harder and harder to draw. Pseudogenes can, at minimum, be expressed despite having such apparent lesions. Moreover, thanks to genomic recoding processes, at least some seeming disablements can be circumvented, leading to the eventual synthesis of a fully-functional peptide. In fact, more recent evidence shows that genomic recoding (in this case, the translational readthrough of premature stop codons) can, at least in yeast genes, no longer be reckoned a rare phenomenon:
‘Our results demonstrate that the presence of a stop codon in a large ORF [open reading frame] may not always correspond to a sequencing error, or a pseudogene, but can be a recoding signal in a functional gene. This emphasizes that genome annotation should take into account the fact that recoding signals could be more frequently used than previously expected.’4
In view of the fact that premature stop codons have traditionally been treated as one of the most obvious supposed ‘gene killers’, this takes on further significance.
Pseudogene function is not easily characterized
Pseudogene nonfunctionality tacitly assumes that any functional peptide synthesized should be the same or very similar to that encoded by the paralogous protein-coding gene. The actual or perceived inability of the pseudogene to direct synthesis of such a peptide is conventionally taken as proof of its ‘junk’ status. However, this long-held premise can no longer be sustained. It is now known that a snail’s pseudogene can direct the synthesis of a useful shortened peptide. This truncated peptide can form a complex with the full-length peptide produced by the paralogous gene, thus functioning as a regulator of the abundance of this full-length protein.5
Nor is it correct to suppose that the pseudogene ‘copy’ of a protein-coding gene must necessarily be translated into any peptide in order to be functional. The snail’s antiNOS (pseudo)gene functions as a regulator of the paralogous protein-coding nNOS gene by producing antisense RNA that forms a duplex with some of the gene’s mRNA, thus regulating the latter’s abundance.6
The recently discovered Makorin1-p1 pseudogene,7,8 the subject of this report, provides further evidence that the pseudogene copy of a protein-coding gene can not only function, but perform a function that is completely unrelated to protein-coding ability. Nor can RNA-only function be stereotyped. As described below, the RNA-only function of the murine Makorin1-p1 pseudogene is completely different from the RNA-only function of the snail’s antiNOS (pseudo)gene, a fact that further underlines the unpredictability of pseudogene function.
The serendipitous discovery of the functional Makorin1-p1 pseudogene
A pseudogene can have one or more ‘disabling lesions’. Examination of the murine Makorin1-p1 pseudogene sequence indicates that it is riddled with insertions, deletions, and numerous nucleotide substitutions relative to the Makorin1 gene.9 The pseudogene also has an in-frame premature stop codon, and its entire 3’ end is missing. If any pseudogene would, according to conventional thinking, be safely assumed to lack function, this particular one would certainly qualify.
Many discoveries in science occur by accident, and the discovery of function in the Makorin1-p1 pseudogene certainly qualifies as one of them. The investigators, Hirotsune et al.,7 were experimenting with the transfer of Drosophila genes into the mouse genome. They noticed that the expression of the mouse’s Makorin1 gene was altered, and eventually realized (and demonstrated by experiment) the fact that they had inadvertently disrupted the regulatory effects of the Makorin1-p1 pseudogene upon the expression of the Makorin1 gene.
The regulatory effect is probably caused by the enhancement of the stability of the mRNA transcribed by the Makorin1 gene.
‘Makorin1-p1 must function as an RNA, as it cannot code for a protein. Protection from mRNA decay of Makorin1 by Makorin1-p1 was easily reproduced by expression constructs in several cell lines and in transgenic mice, suggesting that this type of regulation may be a general phenomenon.’10
Clearly, the pseudogene acts as a ‘switch’ that governs gene expression. There are two possible mechanisms proposed to account for this regulatory effect. Both of these mechanisms involve the pseudogene acting as a ‘sponge’ that absorbs a repressor substance that would otherwise flood the gene and prevent its expression (Figure 1). Unlike the case in the earlier-discussed antiNOS (pseudo)gene,6 the respective RNA species of gene and pseudogene do not interact directly, and no antisense RNA is produced by the Makorin1-p1 pseudogene.9
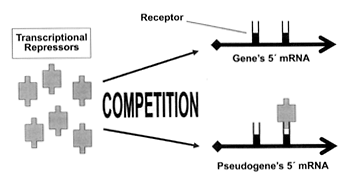
Figure 1. One proposed mechanism by which the Makorin1 gene’s expression is regulated by the Makorin1-p1 pseudogene. Both the gene and pseudogene contain receptor sites (or a similar recognition factor) for transcriptional repressors (RNA-binding peptides). The pseudogene competes for the freely available repressor molecules that would otherwise flood the gene’s receptors and severely inhibit its expression. This frees the gene to synthesize a peptide.
According to the first proposed mechanism, the repressor substance, probably an RNA-binding destabilizing protein, acts not directly on the Makorin1 gene but upon the mRNA that is transcribed by the gene. The repressor substance acts by attaching itself to a receptor (actually, a recognition site) on the mRNA molecule, provoking the rapid degradation of the affected mRNA. Bereft of its mRNA transcript, the gene is effectively shut off, as it cannot direct the synthesis of a peptide. However, the Makorin1-p1 pseudogene is also producing mRNA containing the recognition site, and this competes with the gene for the repressor substance (Figure 1). Relieved of the excessive burden of repressor substance attaching to it, the Makorin1 gene’s mRNA transcript is now stable long enough to be translated into a functional peptide of varying abundance.
Lee proposes a slightly different mechanism of pseudogene-gene interaction.11 As with the first proposed mechanism, recognition is made of the fact that the pseudogene enables the gene to express itself by ‘mopping up’ excess transcription-binding substance. However, instead of binding to receptors on the mRNA of gene and pseudogene in a competitive manner (Figure 1), the transcription-binding substance attaches itself to receptors on the DNA sequence of gene and pseudogene. This can readily be visualized by examining Figure 1 and substituting ‘Gene’s 5´ DNA sequence’ for ‘Gene’s 5´ mRNA’, and substituting ‘Pseudogene’s 5´ DNA sequence’ for ‘Pseudogene’s 5´ mRNA’.
Broad applicability of this discovery
By all accounts, the Makorin1-p1 pseudogene appears to be very crippled. It is commonly supposed that a pseudogene lacks function, relative to its counterpart gene paralog, because it lacks an entire large segment of sequence. The Makorin1-p1 pseudogene demonstrates that this is not the case. Only the first 700 nucleotides of the mRNA transcript of this pseudogene correspond approximately to the mRNA transcript of the paralogous Makorin1 gene. Yet the fragmentary pseudogene DNA segment that is transcribed into this RNA sequence is more than sufficient for the Makorin1-p1 pseudogene to perform its function. This pointedly warns against assuming that even a highly fragmented pseudogene inevitably lacks function.
As elaborated elsewhere,3 there is an entire previously unsuspected ‘hidden world’ of RNA-only functions in the genome. The RNA-only function of the Makorin1-p1 pseudogene opens another window to this long-hidden world:
‘Our findings demonstrate a specific regulatory role of an expressed pseudogene, and point to the functional significance of non-coding RNAs.’12
The functional Makorin1-p1 pseudogene is described as the first known instance of a biological function for any pseudogene.13 Actually, this is not correct. The two earlier-described snail pseudogenes, antiNOS-1 and antiNOS-2, are also examples of functional pseudogenes.5,6 Speaking more broadly, ‘functional pseudogenes’ is a matter of semantics. Whenever a gene sequence is reckoned to lack function, it is labeled a pseudogene. A reversal of this reckoning causes the re-labeling of this sequence as a gene. As noted earlier, there is an entire set of indisputably functional genes that contain pseudogenic features that are circumvented by recoding processes.3 These recoded genes technically qualify as functional pseudogenes. After all, they would not function properly, if at all, was it not for the recoding processes acting upon their pseudogenic features.
It is perhaps ironic that even if the functional Makorin1-p1 pseudogene is taken to be a unique occurrence, it nevertheless retains a broad significance that cannot be minimized:
‘“Pseudogenes” are produced from functional genes during evolution, and are thought to be simply molecular fossils. The unexpected discovery of a biological function for one pseudogene challenges that popular belief.’14
It certainly does. Of course, one does not have to accept the evolutionary spin about functional pseudogenes having been ‘recruited for function’ by evolutionary processes. Instead, we can consider functional pseudogenes as a type of unconventionally behaving gene that, like all genes, were designed to function in their present manner since being specially created.
Conclusions
The functional Makorin1-p1 pseudogene provides another example of a pseudogene that functions by regulating the expression of its gene paralog. The ‘lesions’ that ostensibly prevent the synthesis of a peptide are completely irrelevant to the fact of its function.
Of course, the foregoing discussion hardly exhausts the scope of potential pseudogene function. Pseudogenes, along with a variety of other so-called junk DNA, may have a whole set of functions related to intracellular immunobiology.15 Note that this presents a large avenue of further research that is completely independent from that of pseudogenes as regulators of gene expression.
The variety of known or suspected pseudogene functions discovered to date suggests that pseudogenes as a whole have a wide range of previously unsuspected functions. It is hoped that the evolutionistic ‘pseudogenes are dead gene copies’ mindset that has dominated molecular biology for so long will be decisively abandoned. Now more than ever, the examination of pseudogenes for function should be henceforth conducted in a systematic and large-scale manner.
Footnotes
- Max, E., Plagiarized errors and molecular genetics, <www.talkorigins.org/faqs/molgen/>, last updated 19 March 2002.
- Woodmorappe, J., Are pseudogenes ‘shared mistakes’ between primate genomes? TJ 14(3):55–71, 2000. In his website (Ref. 1), Max tries to belittle the evidence I present by asserting that I focus on ‘rare exceptions’. In actuality, the examples I present have been easily located in the literature, a fact hardly consonant with them being rare exceptions. Finally, some of the studies I cite are based on the analysis of large numbers of pseudogenes. Rare exceptions? Hardly. Other statements by Max appear to be little more than self-serving assertions.
- Woodmorappe, J., Unconventional gene behavior and its relationship to pseudogenes, Proceedings of the Fifth International Conference on Creationism: Technical Symposium Sessions, pp. 505–514, 2003. (1) (2) (3)
- Namy, O., Duchateau-Nguyen, G., et al., Identification of stop codon readthrough genes in Saccharomyces cerevisiae, Nucleic Acids Research 31(9):2289, 2003.
- Woodmorappe, J., Pseudogene function: regulation of gene expression, TJ 17(1):47–52, 2003; p. 49. (1) (2)
- Woodmorappe, Ref. 5, pp. 48–49. (1) (2) (3)
- Hirotsune, S., Yoshida, N., et al., An expressed pseudogene regulates the messenger-RNA stability of its homologous coding gene, Nature 423:91–96, 2003. (1) (2)
- Lee, J.T., Complicity of gene and pseudogene, Nature 423:26–28, 2003.
- Hirotsune et al., Ref. 7, p. 93. (1) (2)
- Hirotsune et al., Ref. 7, p. 96.
- Lee, Ref. 8, p. 28.
- Hirotsune et al., Ref. 7. p. 92.
- Lee, Ref. 8, pp. 27–28.
- Lee, Ref. 8, p. 26.
- Woodmorappe, J., The potential immunological functions of pseudogenes and other ‘junk’ DNA, TJ 17(3):102–108, 2003.